Calculate the molarity of each of the following solutions. CO2 CO2 9 D.

Thermodynamics Does Entropy Increase With A Decrease Or With An Increase In A System S Temperature Physics Stack Exchange
B Dissolution of solid KCl in water.
. Chemistry 22062019 1150 hamidaakter936848. 3A -- 4 B would cause a decrease in entropy. BExplanationEntropy can be decreased only in the cases when liquid is converted to solid.
A When a liquid crystallizes into a solid the molecules attain an ordered state and hence entropy decreases. It increases when the ice melts water is heated water boils water evaporates. CO2 s C02 9 lannahdavies is waiting for your help.
CO2 CO29 D. Leave a Reply Cancel reply. The entropy of a system containing matter is known to increase when the tempeature is increased.
Which of the following processes should lead to an decrease in entropy of the surroundings. Answered Dec 14 2018 by Sahida 800k points selected Dec 14 2018 by faiz. So an example of a reaction that leads to a decrease in entropy is.
Add your answer and earn points. Which of the following processes causes a decrease in the entropy of the system. Which of the following would cause entropy to decrease.
Liquids are in the middle. Mixing of two gases B. Condensation of clouds to form rain.
The reaction increased in order. An increase in numbers of moles from reactants to products eg. Melting of a solid to form a liquid D.
What most determines the entropy of a solid. Solid carbon dioxide will turn into gaseous carbon dioxide. What factors generally determine whether a reaction happens or not.
B Change of water into ice- matter in solid state are more organized. A 2NOg O_2g 2NO_2g You have 3 mol of gas on the left and 2 mol on the right so entropy is decreasing. CO2s CO2 C.
2N2g 3 H2g ------- 2 NH3g Q. The entropy also increases as the pressure or concentration is reduced. Which of the following would cause entropy to decrease.
Chemistry questions and answers. So if moles of solid are reacting to produce gas moles entropy is increasing. CO2 s CO2 C.
Solids have the lowest amount of entropy since atoms are tightly packed together and have little movement. Entropy is indeed a fascinating but somewhat confusing topic. A chemical reaction that has a positive delta G.
Hence entropy is increasing. Your email address will not be published. Hence randomness is decreasing here.
CO2g C02 B. If the reaction involves multiple phases the production of a gas typically increases the entropy much more than any increase in moles of a liquid or solid. Conversely entropy decreases when you have the opposite situations.
Select as many answers as are correct however points will be deducted for incorrect guesses. Solids change to liquid expansion of gas and dissolution of crystal are examples of increasing disorders or increasing entropy. Vaporization of liquid water An exothermic reaction Melting of ice into liquid water Condensation of water vapour Freezing of.
Chemistry 22062019 0540 FedxUp. CO2 g C02 B. The entropy increases whenever heat flows from a hot object to a cold object.
For processes involving an increase in the number of microstates W f W i the entropy of the system increases ΔS 0. Conversely processes that reduce the number of microstates W f W i yield a decrease in system entropy ΔS 0. Generation of a gas from solid or liquid reactants C.
Select one or more. This molecular-scale interpretation of entropy provides a link to the probability that a process will occur as illustrated in the next paragraphs. CO2s C029 Categories Chemistry.
A Boiling water to form steam. In Polymerization the number of molecules present is decreasing so the randomness or entropy. C Dry ice placed in an open vessel.
Which of the following would result in a decrease in entropy. Which of the following reactions would have a decrease in entropy. What does S 0 mean.
Why did southern business leaders want to increase the number of slaves. NH4Clsolid So you see there are more gaseous molecules in the reactant side of the equation. Required fields are marked Comment Name Email Website.
Gases have the highest amount of entropy because they are widespread and can move around all over the place in many different ways. A decrease in the number of moles on the product side means lower entropy. It can be noted that the entropies of gases are much larger than those of condensed phases.
The types of reaction that would decrease the entropy within a cell is dehydration reactions. C Mixing of two gases into one container d Freezing liquid water to form ice e Melting ice to form water. Other questions on the subject.
Question 1 5 pts Which of the following would cause a decrease in the molar entropy of a system. Order and disorder This image shows a series of blue and green squares going from a state of disorder randomness to a state of order a clear repeating pattern. CO2 g -- CO2 L What does S 0 mean in a reaction.
B At 0K the constituent particles are static and entropy is minimum. So randomness is increasing. 2NO2 g -- N2O4 g Which of the following would cause entropy to decrease.
Which of the following would cause entropy to decrease. This is the correct answer. Energy coming from the Sun can decrease the entropy of local systems on Earththat is ΔS syst is negative.
For the following reaction indicate if entropy is increased or decreased. In fact it is so important that the topic of entropy deals with two of the three laws of thermodynamics. What causes an increase in entropy.
An increase in the number of moles on the product side means higher entropy. But the overall entropy of the rest of the universe increases by a greater amountthat is Δ S envir is positive and greater in magnitude. The crystalline structure of the solid.
The system has become more random.

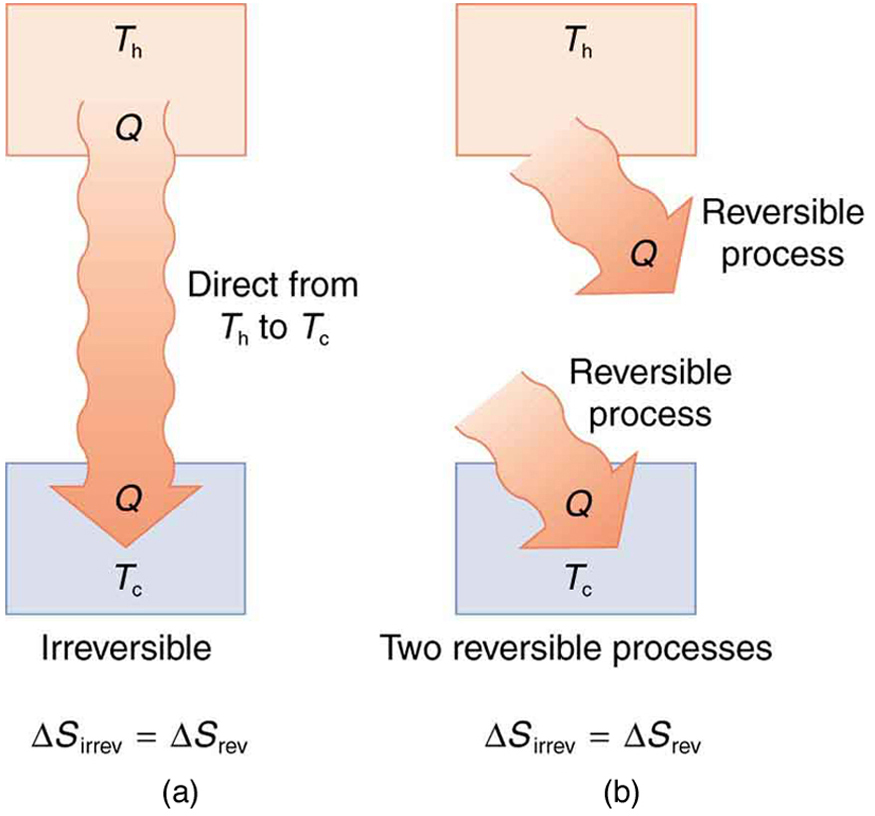
Entropy And The Second Law Of Thermodynamics Disorder And The Unavailability Of Energy College Physics
Increasing Or Decreasing Entropy Youtube
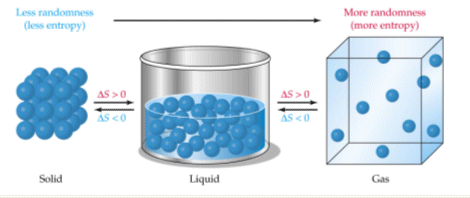
Which Of The Following Processes Shows A Decrease In Entropy Of The System Socratic
0 Comments